Before You Can Write a Chemical Equation
How exercise you Write a Chemical Equation?
All chemical reactions are represented by chemical equations. A chemic equation is a shorthand representation of a chemical reaction using the symbols and formulae of substance involved in the chemic reaction.
The symbols and formulae of the substances (elements or compounds) are arranged to bear witness the reactants and products of a chemic reaction.
A chemical reaction occurs when starting substances react to produce new substances.
(a) Starting substances are called reactants.
(b) New substances formed are chosen products.
In an equation, the reactants are written at the left-hand side whereas the products are written at the correct-hand side.
For example:
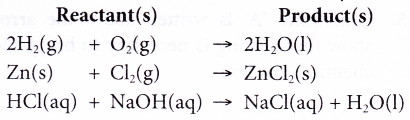
People also ask
- What is the Relative Atomic Mass and Relative Molecular Mass of an Element?
- What is One Mole and How many Particles are in a Mole?
- How exercise you Calculate the Molar Mass of a Substance?
- What is the Molar Book of a Gas at STP?
- How exercise yous know the Social club of Elements in a Chemical Formula
- What is Empirical and Molecular Formula?
Amalgam chemical equations
one. Based on the law of conservation of mass, matter can neither exist created nor destroyed. This means that the numbers of atoms earlier and afterwards a chemical reaction are the same. Therefore, a chemical equation must be balanced.
2. Tabular array below shows how a chemical equation can be constructed.
Table: Constructing a chemical equation.
| Reaction: Iron filings react with copper(Two) chloride to produce iron(3) chloride solution and copper. | |
| Footstep | Explanation and case |
| Place the reactants, products and their formulae. | Reactants: Iron, Atomic number 26 and copper(II) chloride, CuCl2Products : Iron(III) chloride, FeCl3 and copper, Cu |
| Write the primary office of the equation. | Atomic number 26 + CuCl2 ⟶ FeCliii + Cu Reactants Products |
| Determine the number of atoms of each chemical element on both sides of the equation. | Left-hand side Right-manus side Fe atom : 1 Fe cantlet : 1 Cu atom : 1 Cu atom : 1 Cl atom : ii Cl atom : 3 The numbers of atoms are non counterbalanced. |
| Balance the equation past adjusting the coefficients. (Note: Coefficients are the numbers in front of the formulae.) | The Cl atoms are balanced. Atomic number 26 + 3CuCltwo ⟶ 2FeCl3 + Cu As a result, the numbers of Atomic number 26 atoms and Cu atoms are not balanced. The Fe atoms are then balanced. 2Fe + 3CuCI2 ⟶ 2FeCI3 + Cu Lastly, the Cu atoms are counterbalanced. 2Fe + 3CuCI2 ⟶ 2FeCI3 + 3Cu |
| Check that the equation is balanced. | Left-hand side Right-hand side Fe atom : 2 Fe atom : 2 Cu atom: 3 Cu cantlet : three Cl atom : 6 Cl atom : half-dozen Now, the equation is balanced. |
| Put in the land symbol of each substance. | 2Fe(due south) + 3CuCl2(aq) ⟶ 2FeCl3(aq) + 3Cu(s) |
iii. The state symbols (s), (l) and (g) represent the solid, liquid and gaseous states respectively. The symbol (aq) represents an aqueous solution.
4. Sometimes the symbol '↑' is used to betoken the release of a gas.
5. Sometimes '△' is written higher up the arrow to show that heating is necessary to bring about a chemic reaction.
Rules for writing chemical equation:
Certain rules have to exist followed while writing a chemical equation.
- The reactants taking office in the reaction are written in terms of their symbols or molecular formulae on the left-manus side of the equation.
- A plus (+) sign is added betwixt the formulae of the reactants.
- The products of reaction are written in terms of their symbols or molecular formulae on the correct-paw side of the equation.
- A plus (+) sign is added betwixt the formulae of the products.
- In between the reactants and the products an arrow sign (⟶) is inserted to bear witness which mode the reaction is occurring.
A + B ⟶ C + D
In this chemical equation, A and B are the reactants, and C and D are the products. The arrow indicates that the reaction proceeds towards the germination of C and D.
How to Balance Chemical Equations?
The showtime pace in balancing an equation is to count the number of atoms of each chemical element on both sides of the equation. For example, reactants Ten and Y2 react to form a compound XY. The word equation for this reaction would be
X + Y2 ⟶ XY
The number of atoms of elements X and Y in the in a higher place-mentioned equation is shown below.
| Element | Number of atoms in LHS | Number of atoms in RHS |
| X | 1 | i |
| Y | 2 | ane |
To balance Y on both sides, multiply RHS by 2, i.due east.,
X + Ytwo ⟶ 2XY
Now, the number of atoms of Y is counterbalanced merely not the number of atoms of X. Therefore, multiply X on the LHS by two. Thus, the equation becomes
2X + Y2 ⟶ 2XY
This is a counterbalanced equation as the number of atoms of X and Y on both sides is equal.
Keeping these steps in mind, allow us now write the chemical equation for the formation of magnesium oxide.
Step 1: Magnesium burns in oxygen to give magnesium oxide. Hither, the reactants are magnesium and oxygen. The product is magnesium oxide.
Step 2: Thus, the word equation is
Magnesium + Oxygen ⟶ Magnesium oxide
Footstep three: Replacing the names with symbols and formulae, we get the chemical equation every bit
Mg + Otwo ⟶ MgO
Step 4: The number of atoms of the elements are
| Element | Number of atoms in LHS | Number of atoms in RHS |
| Magnesium | 1 | i |
| Oxygen | 2 | ane |
To residuum oxygen on both sides, multiply RHS by 2, i.e.,
Mg + Otwo ⟶ 2MgO
At present, the number of oxygen atoms is balanced but the number of magnesium atoms is not. Therefore, multiply magnesium on the LHS by 2. Thus, the equation becomes
2Mg + O2 ⟶ 2MgO
This is the balanced chemical equation.
Constructing Balanced Chemical Equations
Aim: To construct balanced chemical equations.
Materials: Copper(Ii) carbonate powder, limewater, concentrated hydrochloric acrid, concentrated
ammonia solution, lead(Ii) nitrate solution and potassium iodide solution.
Appliance: Test tubes, stoppers, rubber bung with delivery tube, test tube holder, Bunsen burner and glass tube.
Procedure:
A. Heating of copper(II) carbonate
- One-half a spatula of copper(2) carbonate powder is placed into a examination tube.
- The apparatus is set upward equally shown in Figure.
Effigy: Heating of copper(Ii) carbonate
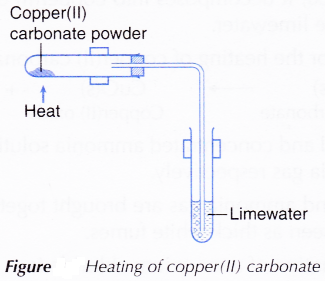
- Copper(II) carbonate is heated and the gas produced is passed through limewater.
- The changes to copper(II) carbonate and limewater are observed.
- When the reaction is completed, the delivery tube is withdrawn from the limewater and the Bunsen burner is removed.
B. Formation of ammonium chloride
- Using a glass tube, three or four drops of full-bodied hydrochloric acid are dropped in a test tube. The exam tube is stoppered and left aside for a few minutes.
- Using a clean glass tube, step 1 is repeated using concentrated ammonia solution.
- Both stoppers are removed and the mouths of the test tubes are brought together as shown in Figure.
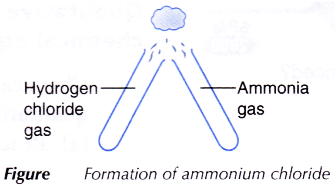
- All observations are recorded.
C. Precipitation of lead(2) iodide
- 2 cmiii of potassium iodide solution is added to two cmiii of pb(Ii) nitrate solution every bit shown in Figure.
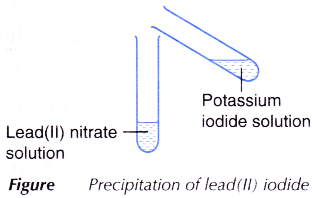
- The mixture is shaken and whatsoever change is observed.
Observations:
| Section | Ascertainment | Inference |
| A | Copper(2) carbonate changes colour from dark-green to black. Limewater turns milky. | Copper(Ii) carbonate decomposes into copper(Ii) oxide, which is blackness in color. Carbon dioxide is released. |
| B | Thick white fumes are produced at the mouth of the test tubes. | The white fumes are solid ammonium chloride. |
| C | A yellow precipitate is produced. | The yellow precipitate is lead(II) iodide. |
Discussion:
- When copper(Ii) carbonate is heated, it decomposes into copper(II) oxide and carbon dioxide. The presence of carbon dioxide is detected by the limewater.
- Therefore, the counterbalanced equation for the heating of copper(II) carbonate is
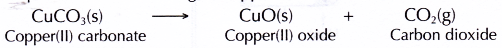
- The full-bodied hydrochloric acid and concentrated ammonia solution are left for a few minutes to produce hydrogen chloride gas and ammonia gas respectively.
- When the hydrogen chloride gas and ammonia gas are brought together, they react to class fine white solids of ammonium chloride. These are seen equally thick white fumes.
- The balanced equation for the germination of ammonium chloride is
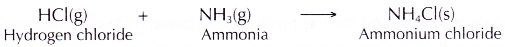
- When the colourless pb(II) nitrate solution is added to the colourless potassium iodide solution, yellow precipitate of atomic number 82(2) iodide is produced. At the aforementioned time, colourless potassium nitrate solution is also produced.
- The counterbalanced equation for the atmospheric precipitation of atomic number 82(II) iodide is

Qualitative and quantitative aspects of chemical equations
- Chemic equations requite us the following qualitative information.
(a) Reactants and products of a chemical reaction.
(b) Physical states of the reactants and products. - Take the following equation as an instance.
2C(s) + O2(g) ⟶ 2CO(chiliad)
From the equation, nosotros know that the reactants are solid carbon and oxygen gas. The product of the reaction is carbon monoxide gas. - Quantitatively, the coefficients in a balanced equation tell us the exact proportions of reactants and products in a chemical reaction.
Take the following equation every bit an example.
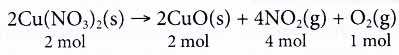
From the equation, we know that 2 moles of copper(II) nitrate decompose into two moles of copper(2) oxide, four moles of nitrogen dioxide gas and 1 mole of oxygen gas. - At the microscopic level, the coefficients in a chemical reaction tell us the number of particles ane involved in the reaction.
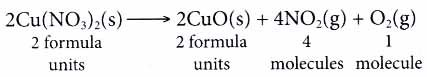
- A chemical equation serves as an important ; communicative tool for chemists.
(a) A chemical equation precisely describes a chemical reaction.
(b) Chemists apply chemic equations to solve quantitatively-related issues.
Note:
- Qualititatively, hydrogen gas reacts with oxygen gas to give water.
- Qualitatively, 2 molecules (or two moles) of hydrogen gas react with ane molecule (or i mole) of oxygen gas to requite 2 molecules (or 2 moles) of water.
Source: https://www.aplustopper.com/chemical-equations/
Post a Comment for "Before You Can Write a Chemical Equation"